Turn-Key EEG Services to Support Your Clinical Trials
Stratus is a leading provider of EEG services for clinical trials with experience in pharmaceutical, medical device, and investigator led trials. We make adding EEG data to your clinical trial turnkey for you, your investigators, and study participants.
We bring expertise from more than 150,000 studies to help you successfully obtain the data needed to support your trial and determine treatment impact. With a U.S. and European service area, equipment, and software that meets HIPAA, FDA, MDR and GDPR requirements, including advanced security features with 256-bit encryption and full audit capabilities, we check all the boxes so you don’t have to.

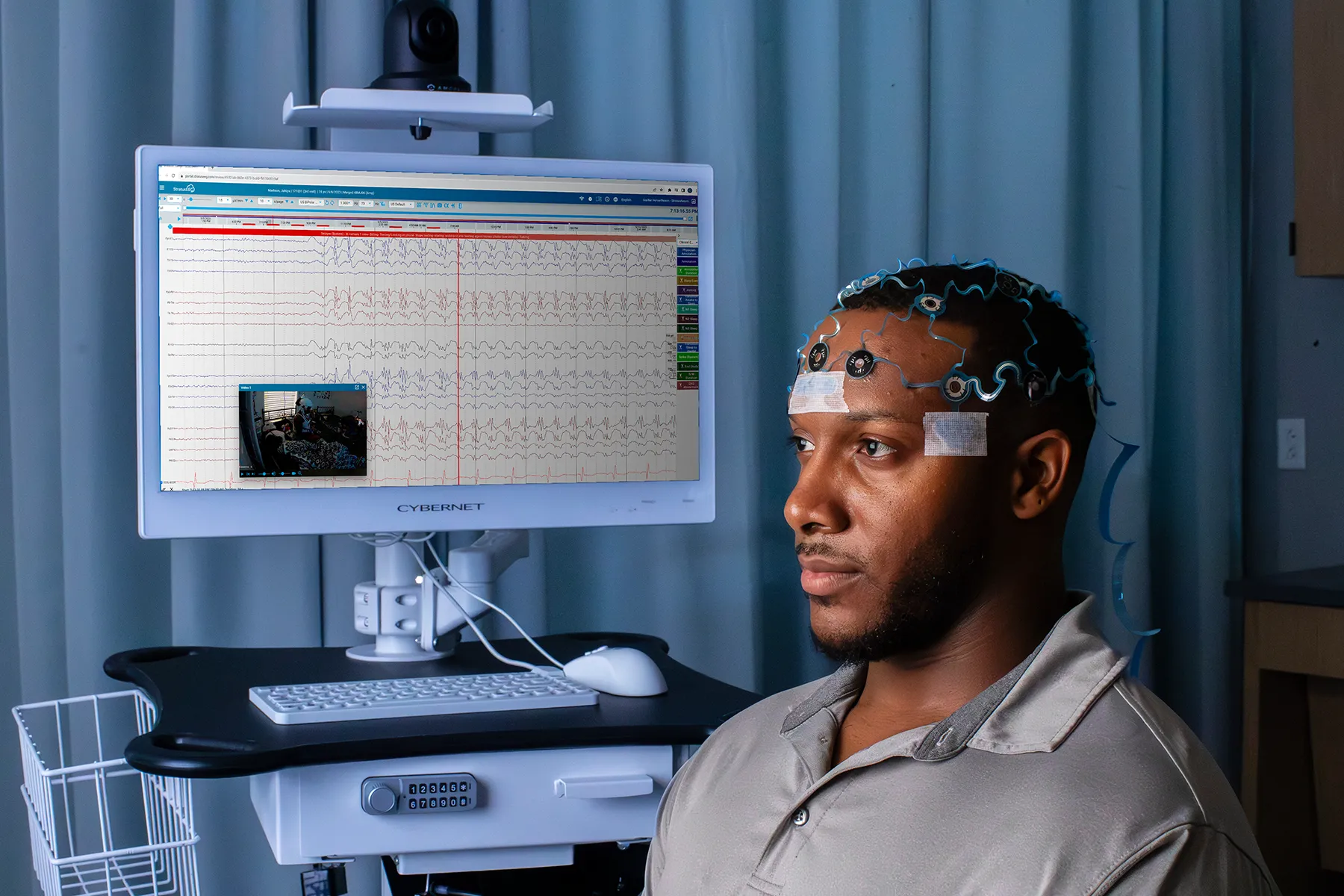
Standardize Your EEG Clinical Trial Methodology
In multi-site clinical trials, methodological consistency is the foundation of trustworthy data. Our processes help ensure data integrity and uniformity for increased confidence in study results. Standardized EEG equipment and set-up methodology for data acquisition are used across sites, and our EEG Technologists have standardized EEG and protocol-specific training.
Stratus’ EEG Services for Clinical Trials
Full-Service EEG Testing (with and without video)
- Routines - stimulation procedures upon request (photic, hyperventilation, or sponsor-specified)
- Long-term EEG - set-up available in-home or on-site
- Remote monitoring services - intermittent or continuous monitoring for vigilance monitoring and quality control
- ECG and PSG also available concurrent with EEG
- Adult and pediatric experience, including subjects with developmental challenges
- Decentralized clinical trials (DCTs), on-site, or in-clinic
Web-Based EEG Data Access with Private Segmentation & Secure Transfer
- Full study data provided with the option for annotation and pruning by R. EEG T.s
- Access to StratusEEGTM best-in-class, cloud-based software to review and interpret data from any internet-connected device
- Optional: augmented EEG safety reads
- Data quality checks and secure API data transfer

Trial Design & Coordination Support
- Assist with the design of EEG testing protocols
- Develop guidance, source, and CFR documentation
- Provide a dedicated project manager and data manager, if needed
- Optional: centralized EEG interpretation through our board-certified national reader group



%201.svg)